R&D
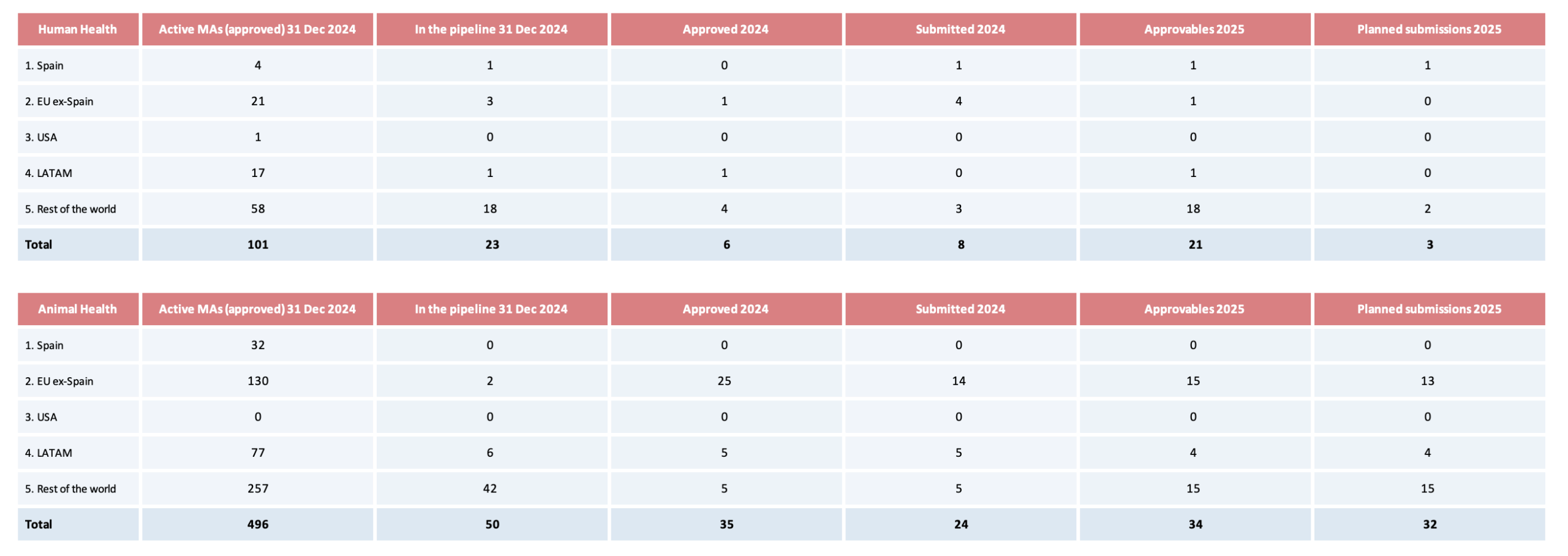
Marketing authorization activities in each division during 2024
CONTACT
LABIANA HEALTH, S.A.
TIN A87992616
Avenida Europa, 34 1ºD
28023 Pozuelo de Alarcón (Madrid)
Avenida Europa, 34 1ºD
28023 Pozuelo de Alarcón (Madrid)
CONTACT FOR INVESTORS
Email: investors@labiana.com
Phone: (+34) 91 991 26 28
Phone: (+34) 91 991 26 28



